Frequently asked questions
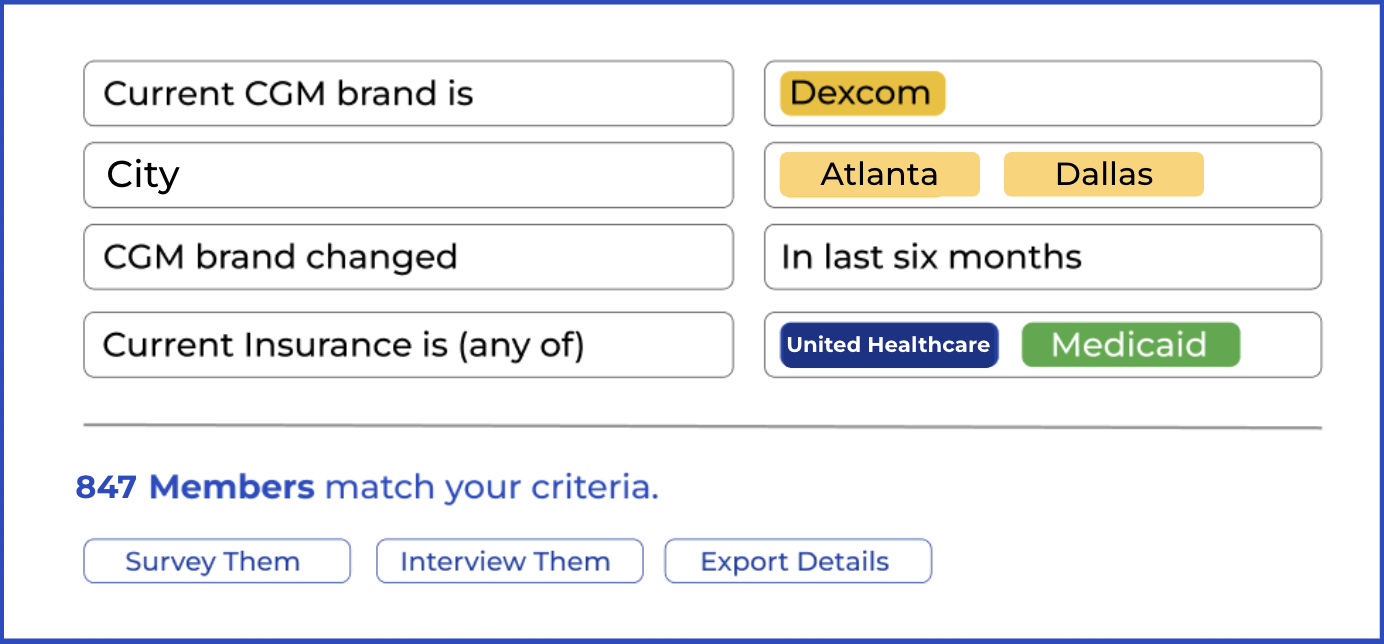
Thrivable specializes in recruiting patients for diabetes and obesity studies, with access to over 70,000 engaged individuals living with diabetes and related conditions. We have conducted a variety of research studies for diverse purposes, including obtaining FDA medical device approval.
Thrivable provides recruitment, screening, scheduling, gap analysis, quota management, dedicated staff support, and incentive fulfillment for your human factors and medical device studies.
Yes, we manage incentive distribution, keeping participants motivated and engaged while allowing your team to focus on the study itself.
While we don’t directly offer hosting locations, we collaborate closely with trusted site vendors to ensure all logistical details are seamlessly managed, so you can focus on the study itself without added concerns.
Our recruitment process is designed for speed, with patients typically scheduled within two weeks for remote studies and around four weeks for in-person studies.
We employ best-in-class fraud prevention and collect condition-specific data that is regularly updated to provide only high-quality, motivated patients who meet your study criteria.
We are experts in what we do, not generalists. With over 100 years of combined experience, our team brings deep expertise in diabetes and obesity recruitment, allowing us to connect you with the right patients faster and more reliably.
We prioritize patient privacy, following strict data protection protocols and compliance standards to ensure the safety and confidentiality of all patient information. View our privacy policy to learn more.
Thrivable offers both project-based and subscription pricing options. Our subscription model provides flexibility and cost savings as your research needs expand, with the added benefit of seamlessly combining qualitative, quantitative, and human factors efforts into a single, cohesive package—all at preferred pricing.